Programs Prostate
Prostate Active Surveillance
Optimizing treatment appropriateness for patients with favorable risk prostate cancer
What is Active Surveillance
- Our goal is to provide guidance for determining who should consider active surveillance (AS) and steps for how to perform surveillance and ultimately enhance the use of follow-up testing for better management of patients on surveillance.
- Optimize the consideration of active surveillance for patients with favorable risk prostate cancer.
- Increase rates of follow-up testing for better management of patients on surveillance.
What we do:
- Enhance the management of men with favorable-risk prostate cancer through measuring our progress and providing feedback for improvement to our members.
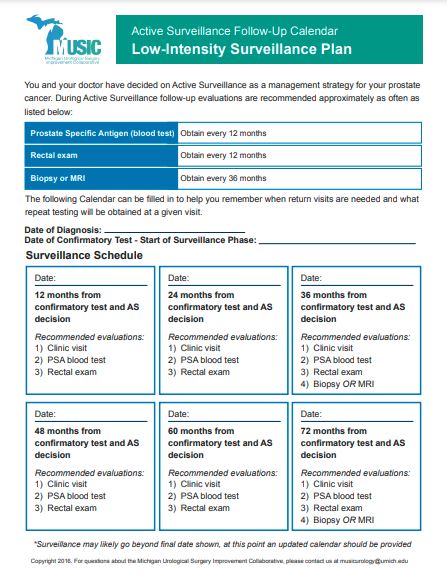
Roadmap for Management of Men With Favorable-Risk Prostate Cancer
- MUSIC developed a systematic approach, or roadmap, for the management of men with favorable-risk, early-stage prostate cancer.
- Defined as patients with early-stage tumors with a Gleason Score of 6 or less, as well as select patients with low-volume Gleason Score 3+4=7 cancer.
- The MUSIC AS Roadmap is available in the Provider Resources section at the bottom of the page. Care is divided into two distinct phases that begin at the time of diagnosis:
- Consideration Phase: Steps to take while considering AS and
- Surveillance Phase: A roadmap for how to perform AS.
- The Roadmap is meant to serve as a framework for determining when a patient is a candidate for surveillance, final decisions about the best treatment option for any patient remain, of course, at the discretion of the patient and their urologist.
Roadmap Implementation
- Please click on the video below to access a MUSIC webinar which includes a discussion on the active surveillance roadmap background, content, etc., additional patient educational resources, as well as an interactive Q & A session.
Management of Prostate Cancer Patients on Active Surveillance: Primary Care Provider Role
- Primary Care Providers (PCPs), in addition to urologists, play a critical role in ensuring prostate cancer patients on Active Surveillance receive the recommended follow-up testing. To learn more and access PCP specific resources, please click here.